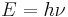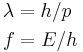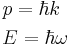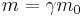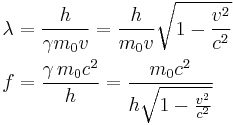Matter wave
In quantum mechanics, a matter wave or de Broglie wave ( /dəˈbrɔɪ/) is the wave (wave–particle duality) of matter.The theory was proposed by Louis de Broglie in 1924 in his PhD thesis.[1]. The de Broglie relations show that the wavelength is inversely proportional to the momentum of a particle and is also called de Broglie wavelength. Also the frequency of matter waves, as deduced by de Broglie, is directly proportional to the particle's total energy, i.e. the sum of particle's Kinetic energy and rest energy.
Contents |
Historical context
At the end of the 19th century, light was thought to consist of waves of electromagnetic fields which propagated according to Maxwell’s equations, while matter was thought to consist of localized particles (See history of wave and particle viewpoints ). This division was challenged when, in his 1905 paper on the photoelectric effect, Albert Einstein postulated that light was emitted and absorbed as localized packets, or “quanta” (now called photons). These quanta would have an energy
where 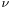 is the frequency of the light and h is Planck’s constant. Einstein’s postulate was confirmed experimentally by Robert Millikan and Arthur Compton over the next two decades. Thus it became apparent that light has both wave-like and particle-like properties. De Broglie, in his 1924 PhD thesis, sought to expand this wave-particle duality to all particles:
is the frequency of the light and h is Planck’s constant. Einstein’s postulate was confirmed experimentally by Robert Millikan and Arthur Compton over the next two decades. Thus it became apparent that light has both wave-like and particle-like properties. De Broglie, in his 1924 PhD thesis, sought to expand this wave-particle duality to all particles:
"When I conceived the first basic ideas of wave mechanics in 1923-24, I was guided by the aim to perform a real physical synthesis, valid for all particles, of the coexistence of the wave and of the corpuscular aspects that Einstein had introduced for photons in his theory of light quanta in 1905".[2]
In 1926, Erwin Schrödinger published an equation describing how this matter wave should evolve — the matter wave equivalent of Maxwell’s equations — and used it to derive the energy spectrum of hydrogen. That same year Max Born published his now-standard interpretation that the square of the amplitude of the matter wave gives the probability to find the particle at a given place. This interpretation was in contrast to De Broglie’s own interpretation, in which the wave corresponds to the physical motion of a localized particle.
The de Broglie relations
The de Broglie equations relate the wavelength λ to the momentum p, and frequency f to the total energy E (including its rest energy) of a particle:[3]
where h is Planck's constant. The two equations can be equivalently written as
using the definitions 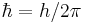 is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),
is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"), 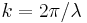 is the angular wavenumber, and
is the angular wavenumber, and 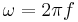 is the angular frequency. In each pair, the second is also referred to as the Planck-Einstien relation, since it was also proposed by Planck and Einstein.
is the angular frequency. In each pair, the second is also referred to as the Planck-Einstien relation, since it was also proposed by Planck and Einstein.
Using the relativistic mass formula from special relativity
allows the equations to be written as[4]
where m0 is the particle's rest mass, v is the particle's velocity, 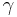 is the Lorentz factor, and c is the speed of light in a vacuum. See group velocity for details of the derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity (equal to the product of the particle's frequency and its wavelength). In the case of a non-dispersive medium, they happen to be equal, but otherwise they are not.
is the Lorentz factor, and c is the speed of light in a vacuum. See group velocity for details of the derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity (equal to the product of the particle's frequency and its wavelength). In the case of a non-dispersive medium, they happen to be equal, but otherwise they are not.
Experimental confirmation
Elementary particles
In 1927 at Bell Labs, Clinton Davisson and Lester Germer fired slow-moving electrons at a crystalline nickel target. The angular dependence of the reflected electron intensity was measured, and was determined to have the same diffraction pattern as those predicted by Bragg for x-rays. Before the acceptance of the de Broglie hypothesis, diffraction was a property that was thought to be only exhibited by waves. Therefore, the presence of any diffraction effects by matter demonstrated the wave-like nature of matter. When the de Broglie wavelength was inserted into the Bragg condition, the observed diffraction pattern was predicted, thereby experimentally confirming the de Broglie hypothesis for electrons.
This was a pivotal result in the development of quantum mechanics. Just as the photoelectric effect demonstrated the particle nature of light, the Davisson-Germer experiment showed the wave-nature of matter, and completed the theory of wave-particle duality. For physicists this idea was important because it means that not only can any particle exhibit wave characteristics, but that one can use wave equations to describe phenomena in matter if one uses the de Broglie wavelength.
Since the original Davisson-Germer experiment for electrons, the de Broglie hypothesis has been confirmed for other elementary particles.
The wavelength of a thermalized electron in a non-metal at room temperature is about 8 nm.
Neutral atoms
Experiments with Fresnel diffraction[5] and specular reflection[6][7] of neutral atoms confirm the application of the de Broglie hypothesis to atoms, i.e. the existence of atomic waves which undergo diffraction, interference and allow quantum reflection by the tails of the attractive potential.[8] Advances in laser cooling have allowed cooling of neutral atoms down to nanokelvin temperatures. At these temperatures, the thermal de Broglie wavelengths come into the micrometre range. Using Bragg diffraction of atoms and a Ramsey interferometry technique, the de Broglie wavelength of cold sodium atoms was explicitly measured and found to be consistent with the temperature measured by a different method.[9]
This effect has been used to demonstrate atomic holography, and it may allow the construction of an atom probe imaging system with nanometer resolution.[10][11] The description of these phenomena is based on the wave properties of neutral atoms, confirming the de Broglie hypothesis.
Waves of molecules
Recent experiments even confirm the relations for molecules and even macromolecules, which are normally considered too large to undergo quantum mechanical effects. In 1999, a research team in Vienna demonstrated diffraction for molecules as large as fullerenes.[12] The researchers calculated a De Broglie wavelength of the most probable C60 velocity as 2.5 pm. More recent experiments prove the quantum nature of molecules with a mass up to 6910 amu,[13] and even macroscopic droplets. [14] In general, the De Broglie hypothesis is expected to apply to any well isolated object.
Spatial Zeno effect
The matter wave leads to the spatial version of the Zeno effect. If an object (particle) is observed with frequency 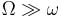 in a half-space (say,
in a half-space (say, 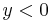 ), then this observation prevents the particle, which stays in the half-space
), then this observation prevents the particle, which stays in the half-space 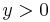 from entry into this half-space
from entry into this half-space 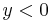 . Such an "observation" can be realized with a set of rapidly moving absorbing ridges, filling one half-space. In the system of coordinates related to the ridges, this phenomenon appears as a specular reflection of a particle from a ridged mirror, assuming the grazing incidence (small values of the grazing angle). Such a ridged mirror is universal; while we consider the idealised "absorption" of the de Broglie wave at the ridges, the reflectivity is determined by wavenumber
. Such an "observation" can be realized with a set of rapidly moving absorbing ridges, filling one half-space. In the system of coordinates related to the ridges, this phenomenon appears as a specular reflection of a particle from a ridged mirror, assuming the grazing incidence (small values of the grazing angle). Such a ridged mirror is universal; while we consider the idealised "absorption" of the de Broglie wave at the ridges, the reflectivity is determined by wavenumber 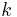 and does not depend on other properties of a particle.[7]
and does not depend on other properties of a particle.[7]
See also
- Atom optics
- Atomic de Broglie microscope
- Atomic mirror
- Bohr model
- Faraday wave
- Quantum reflection
- Ridged mirror
- Theoretical and experimental justification for the Schrödinger equation
- Thermal de Broglie wavelength
- Zeno effect
References
- ^ L. de Broglie, Recherches sur la théorie des quanta (Researches on the quantum theory), Thesis (Paris), 1924; L. de Broglie, Ann. Phys. (Paris) 3, 22 (1925).
- ^ Louis de Broglie "The Reinterpretation of Wave Mechanics" Foundations of Physics, Vol. 1 No. 1 (1970)
- ^ Resnick, R.; Eisberg, R. (1985). Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles (2nd ed.). New York: John Wiley & Sons. ISBN 047187373X.
- ^ Holden, Alan (1971). Stationary states. New York: Oxford University Press. ISBN 0195014979.
- ^ R.B.Doak; R.E.Grisenti, S.Rehbein, G.Schmahl, J.P.Toennies2, and Ch. Wöll (1999). "Towards Realization of an Atomic de Broglie Microscope: Helium Atom Focusing Using Fresnel Zone Plates". Physical Review Letters 83: 4229–4232. Bibcode 1999PhRvL..83.4229D. doi:10.1103/PhysRevLett.83.4229.
- ^ F. Shimizu (2000). "Specular Reflection of Very Slow Metastable Neon Atoms from a Solid Surface". Physical Review Letters 86: 987–990. Bibcode 2001PhRvL..86..987S. doi:10.1103/PhysRevLett.86.987.
- ^ a b D. Kouznetsov; H. Oberst (2005). "Reflection of Waves from a Ridged Surface and the Zeno Effect". Optical Review 12: 1605–1623. Bibcode 2005OptRv..12..363K. doi:10.1007/s10043-005-0363-9.
- ^ H.Friedrich; G.Jacoby, C.G.Meister (2002). "quantum reflection by Casimir–van der Waals potential tails". Physical Review A 65: 032902. Bibcode 2002PhRvA..65c2902F. doi:10.1103/PhysRevA.65.032902.
- ^ Pierre Cladé; Changhyun Ryu, Anand Ramanathan, Kristian Helmerson, William D. Phillips (2008). "Observation of a 2D Bose Gas: From thermal to quasi-condensate to superfluid". arXiv:0805.3519.
- ^ Shimizu; J.Fujita (2002). "Reflection-Type Hologram for Atoms". Physical Review Letters 88 (12): 123201. Bibcode 2002PhRvL..88l3201S. doi:10.1103/PhysRevLett.88.123201. PMID 11909457.
- ^ D. Kouznetsov; H. Oberst, K. Shimizu, A. Neumann, Y. Kuznetsova, J.-F. Bisson, K. Ueda, S. R. J. Brueck (2006). "Ridged atomic mirrors and atomic nanoscope". Journal of Physics B 39: 1605–1623. Bibcode 2006JPhB...39.1605K. doi:10.1088/0953-4075/39/7/005.
- ^ Arndt, M.; O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, A. Zeilinger (14 October 1999). "Wave-particle duality of C60". Nature 401 (6754): 680–682. Bibcode 1999Natur.401..680A. doi:10.1038/44348. PMID 18494170.
- ^ Gerlich, S.; S. Eibenberger, M. Tomandl, S. Nimmrichter, K. Hornberger, P. J. Fagan, J. Tüxen, M. Mayor & M. Arndt (05 April 2011). "Quantum interference of large organic molecules". Nature Communications 2 (263). Bibcode 2011NatCo...2E.263G. doi:10.1038/ncomms1263.
- ^ "Bouncing droplets, the role of deformations". http://bictel.ulg.ac.be/ETD-db/collection/available/ULgetd-09262011-010800/unrestricted/2011_Terwagne_these.pdf.
Further reading
- Broglie, Louis de, The wave nature of the electron Nobel Lecture, 12, 1929
- Tipler, Paul A. and Ralph A. Llewellyn (2003). Modern Physics. 4th ed. New York; W. H. Freeman and Co. ISBN 0-7167-4345-0. pp. 203–4, 222–3, 236.
- Web version of Thesis, translated by Kracklauer (English)
- Zumdahl, Steven S. (2005). Chemical Principles (5th ed.). Boston: Houghton Mifflin. ISBN 0618372067.
- An extensive review article "Optics and interferometry with atoms and molecules" appeared in July 2009: http://www.atomwave.org/rmparticle/RMPLAO.pdf.